Monoclonal Antibody Production Details:
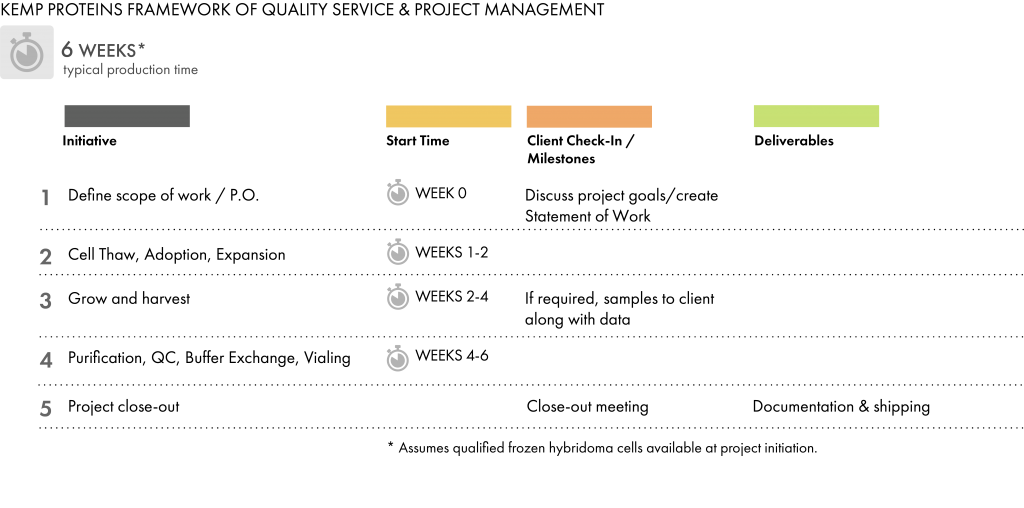
We accept hybridoma cell lines directly from clients or from third-party sources, or we can develop hybridoma cell lines for the project. If the hybridoma cell line is sourced from the client or from a third party, we require:
- Mycoplasma-negative confirmation
- Species of origin
- Known productivity levels
- Known required media components
- Antibody isotype
- Two vials of frozen cells
Our purification group provides both fully automated (BioRad NGC & GE AKTAPilot) and manual purification techniques performed in an ISO 7 / Class 10,000 environment. Our team is experienced with most standard purification media, including Protein A, Protein G, Protein A/G, and LigaTrap. Final buffer requirements are specified by the client.
For standard hybridoma productions, we routinely perform:
- Protein concentration / total antibody
- Antibody isotype
- pH/ buffer type
- Purity
- Aggregation
- Endotoxin levels
In addition, we consider other quality control assay methods requested by clients—we have qualified various client-driven QC methods and have provided the data as part of the release criteria.
We store final product in a cold room facility with generator back-up until it is shipped. We package and ship (or drop-ship) final product according to client specifications.
Kemp Proteins offers the following value-added services: