The Details
Our highly automated process is designed to provide you with a cell bank containing cells that have a similar high yield of target recombinant antibody and that have a robust growth in chemically defined culture media. The process employed by our experienced team can commence with either the genetic sequence for the selected mAb or with a sample of the mAb itself. We use royalty-free proprietary vectors to transfect target cells. We have experience of working with CHO and HEK-293 cells for creation of Stable Cell Banks. Using a set of pre-designed steps, we will progress your project:
We will generate a Research Stable Cell Bank that after qualification can be developed as a cGMP Master Cell Bank for large scale commercial production of your target antibody.
Use our quick quote form to request a quote
Client communication: We consider this vital to maximizing the potential for a successful project outcome. Our initial discussions are information gathering against a set of questions. The information gained enables us to generate a statement of work, a “project design”, that is shared with you. Together we refine the plan until you are in agreement that we have captured the required specifications and deliverables for the project together with and nuances that may be particular to your project. Throughout the development of the Stable Cell Line we will supply regular updates through conference calls or information download through electronic means such as “Box”.
Project Commencement: The recombinant antibody (rAb) project begins when you provide us the genetic sequence for the selected monoclonal antibody (mAb) or the mAb itself so that we can obtain the required genetic sequence.
Project Approach: Our standard approach to cell line development is outlined below. We have found this an efficient and effective process to achieve the desired outcome of our clients.
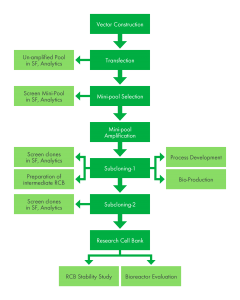
Cell Line Development Flowchart
Kemp Proteins has a strategy for the molecular cloning of the gene(s) from synthetic deoxyribonucleic acid (DNA) into expression vectors for transfection into CHO or HEK-293 cells.
The goal is to provide you with cells that grow to high density in chemically defined media, are stable over 30 passages and have expression levels that meet the acceptance criteria of 2g/L to 4g/L. Kemp will perform several activities for the development of clonal cell lines to meet this goal.
Transfection and Mini-pool Selection and Amplification. Kemp Proteins directs the synthesis of the gene(s) and clones the gene(s) for antibody heavy and light chains into Kemp Proteins’ expression vectors. Kemp Proteins then confirms the genetic structure of the proprietary expression vector(s) through restriction mapping and through DNA sequence analysis. Vector DNA(s) required for electroporation is generated and electroporations are performed into the Kemp Proteins host cell line. After electroporation recovery, each transfected culture is dispersed into 96-well plates and placed under appropriate selection conditions. Mini-pools are subjected to selection in the presence of Methotrexate (MTX) and allowed to grow to near confluence. The remaining cells from each transfection not plated are pooled and selected in a serum-free medium in absence of MTX. Kemp Proteins evaluates each mini-pool by using an enzyme-linked immunosorbent assay (ELISA) or bio-layer interferometry (BLI). The mini-pools with the highest level of ELISA response are expanded and screened for expression by ELISA or BLI with the best mini-pools (up to 20) selected for expansion in shake flasks.
Mini-pools are analyzed for expression in shake flasks using a fed-batch process and evaluated at regular intervals. On each evaluation day, the viability, viable cell density, and titer are measured and a retention sample is archived. Metabolites are measured every second day or daily based on glucose and glutamine consumptions, and cultures are supplemented with glucose and glutamine, as needed. Titer analysis is performed and conditioned media from the top five expresser mini-pools and un-amplified pool is analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and/or Western Blot analysis. Based on titer data, super-pools are prepared by combining cells from the top mini-pools presenting similar titers. The number of super-pools generated and, which mini-pools are selected, is at the sole discretion of Kemp Proteins. The super-pools are expanded and safety freezes prepared.
First Subcloning and Screening of the Super-Pools. Kemp Proteins clones the cells from up to 10 super-pools by using a ClonePix2 instrument (Molecular Devices) with an appropriate animal component–free semi-solid medium. Cells from each super-pool are seeded in a minimum of two 6-well plates and when colonies have grown to an appropriate size, they are imaged. Colonies meeting selection criteria for colony separation and non-irregular colony shape are selected and placed into 96-well plates. When the selected clones have reached a suitable cell density, the titer is determined using ELISA, and the 120 highest expressing candidates are selected for advancement. These clones are expanded in 24-well plates and the titer is determined with the best 36 clonal cell lines selected for further expansion into shake flasks. The above work will be summarized in a set of PowerPoint slides and provided to LBR.
Second Round of Subcloning and Shake Flask Screening of Primary Clones. Kemp Proteins performs a second cloning for up to 12 clones selected during the 24-well plate screening to provide a higher probability of clonality following the ClonePix process. Kemp Proteins subclones each of the selected clones into five 96-well plates with cells seeded at 1 cell/well, which is verified by imaging the wells. All of the resultant subclones are tested with by ELISA, and approximately 90 subclones from the 12 parental clones expanded to 24-well plates. These selected clones are evaluated for titer by ELISA or BLI. Based on the titer data, the top 20 subclones, representing at least three parental clones are expanded to shake flasks. Clonality assurance is provided through a combination of initial dilution plus a time-course of imaging using the CloneSelect Imager (Molecular Devices). In parallel with the second round of subcloning, the 36 primary clones are also analyzed for expression in shake flasks using a fed-batch process and evaluated at regular intervals. On each evaluation day, the viability, viable cell density, and titer are measured and a retention sample is archived. Metabolites are measured every second day or daily based on glucose and glutamine consumptions, and cultures are supplemented with glucose and glutamine, as needed. Titer analysis is performed and conditioned media from the top ten expresser clones are analyzed by Kemp Proteins by SDS-PAGE. Kemp Proteins then evaluates the data and selects the 10 cell lines for intermediate Research Cell Banks (iRCBs).
Evaluation of the Top Subclones in Fed-Batch Shaker Flasks and Cell Bank Construction. Kemp Proteins evaluates all clonal cell lines generated in the previous process for expression in shake flasks by using a fed-batch process. On each evaluation day, the viability, viable cell density, and titer are measured and a retention sample is archived. Metabolites are measured on every second day or daily based on glucose and glutamine consumption, and cultures are supplemented with glucose and glutamine, as needed.
Kemp Proteins reviews the data on the clonal cell lines and selects three clones for research cell bank (RCB) generation and testing. Kemp Proteins prepares a 36-vial RCB with 1×107 cells/mL per vial for each of the three selected clonal cell lines and ships several vials of the lead RCB to LBR after successful completion of sterility, mycoplasma, gene sequencing, and endotoxin testing.
Kemp Proteins thaws two vials from each of the RCBs and monitors the cell viability and growth rate of the cultures over three passages to test the quality of the freeze. Additionally, Kemp Proteins performs generational expression stability on each of the RCBs by thawing a vial and passaging the cells for 60 in the presence and absence of the selective agent(s). The cells are passaged on a routine schedule, and the product titer is determined at regular intervals. Kemp Proteins prepares a draft cell line development report, for client to provide comments and then the report is finalized and submitted to client.
Bioreactor Evaluation. Kemp Proteins evaluates the expression of the RCBs by thawing one vial from each bank and evaluating growth in a 5–L process controlled, stirred tank bioreactor run. The process will utilize Kemp Proteins’ standard platform and include daily metabolite monitoring, a standard feed schedule with glucose supplementation as needed and periodic viability and cell density determinations. Once the cultures reach < 80% cell viability, the cell culture is harvested by clarification of the supernatant using depth filtration followed by purification using protein A (i.e., MabSelect Sure or equivalent) column chromatography.
The purified protein will be analyzed using SDS-PAGE (reduced and non-reduced), IEF and protein sequence. Purified rAb is formulated at 50 mg/mL in phosphate-buffered saline (PBS), pH 7.4 or in a client-designated buffer.
The program outlined herein will be performed under Kemp’s ISO9001:2015 compliant Quality Management System (QMS). This QMS based on risk assessment meets all the requirements of ISO 9001:2015.